
|

|

|

|
|
This article may be reprinted free of charge provided 1) that there is clear attribution to the Orthomolecular Medicine News Service, and 2) that both the OMNS free subscription link http://orthomolecular.org/subscribe.html and also the OMNS archive link http://orthomolecular.org/resources/omns/index.shtml are included. FOR IMMEDIATE RELEASE
The VICTAS Trial: Designed to Fail
|
| Box 1 Rules for individual clinical studies of nutrient effects. | Box 2 Rules for study inclusion in systematic reviews and meta-analyses. |
| 1. Basal nutrient status must be measured, used as an inclusion criterion for entry into study, and recorded in the report of the trial.
2. The intervention (i.e., change in nutrient exposure or intake) must be large enough to change nutrient status and must be quantified by suitable analyses. 3. The change in nutrient status produced in those enrolled in the trials must be measured and recorded in the report of the trial. 4. The hypothesis to be tested must be that a change in nutrient status (not just a change in diet) produces the sought-for effect. 5. Co-nutrient status must be optimized in order to ensure that the test nutrient is the only nutrition related, limiting factor in the response. |
1. The individual studies selected for review or meta-analysis must have met the criteria listed in Box 1 for nutrient trials.
2. All included studies must have started from the same or similar basal nutrient status values. 3. All included studies must use the same or closely similar doses. 4. All included studies must have used the same chemical form of the nutrient and, if foods are used as the vehicle for the test nutrient, all studies must have employed the same food matrix. 5. All included studies must have the same co-nutrient status. 6. All included studies must have had approximately equal periods of exposure to the altered intake. |
The VICTAS Trial [1] satisfied none of these 5 rules for conducting nutrient research.
Recent research has shown the importance of vitamin C in sepsis and other acute life-threatening illnesses. Vitamin C has a multitude of essential for life effects within the human body, and due to its short half-life, is often the rate limiting factor in these biochemical processes. It is the primary extracellular antioxidant, and is important for scavenging damaging electron radicals. At very high levels it is involved in redox regulation, is a pro-oxidant, and can cause DNA and/or protein damage. This is useful in the treatment of cancer. It is an essential co-factor in the synthesis of catecholamines, vasopressin, steroids, neuropeptides and some neurotransmitters. It is also essential in the synthesis of collagen and elastin -- which are important molecules throughout the body, including in arteries and joints. Vitamin C is also important for epigenomic regulation of genes and is necessary for many cell types of the adaptive immune system. These biochemical functions are essential for improved immune cell function, endothelial cell function, hemodynamics (circulatory function), and wound healing.
Stress, including cold temperatures, toxins, infections, and trauma greatly increase the cellular demand for vitamin C, and disrupt the body's ability to recycle oxidized vitamin C (dehydroascorbic acid or DHAA) back into the reduced form of vitamin C (ascorbic acid). Vitamin C has a short half life in the body (minutes to hours). In 2008, the prestigious journal Cell published the discovery that the red blood cells of humans (and other mammals unable to produce vitamin C) express a large number of GLUT1 transporters - more GLUT1 than on any other human cell type. [25] These GLUT1 transporters are apparently misnamed, as they might more properly be called DHAA1 transporters. The human RBC GLUT1 transporter is co-expressed with the protein stomatin which switches it into a DHAA transporter rather than a glucose transporter. [25] The result is 20-30 trillion red blood cells in healthy humans circulating through miles of blood vessels "soaking up" DHAA and - if adequate levels of the selenoprotein glutathione peroxidase are present in the red blood cells - reducing the DHAA back to AA and sending it back into the blood. A similar recycling system is present in the brain between astrocytes and tanycytes. [26] This supports the concept that keeping the blood, vasculature, and brain bathed in adequate ascorbic acid is important.
Humans in acute distress from toxins, viruses, and bacteria have been successfully treated with high dose vitamin C injections for over 70 years. Recent studies have shown a synergistic benefit to endothelial cells when vitamin C and cortisol are injected into blood vessels simultaneously. Decades of experience have underscored the importance of early intervention, and increasing the dose and duration as needed to neutralize the acidosis and/or toxins. [27-53]
Below is a graph courtesy of Dr. Paul E Marik of an ICU patient's c-reactive protein level (biomarker of inflammation) during 3g IVC and a corticosteroid co-administration every 6 hours for 96 hours, stopping the treatment, and then resuming the treatment. Continued vitamin C treatment until full recovery, tapering from IV to oral administration as the patient recovers, is important. It takes ongoing administration of vitamin C to achieve and maintain the tissue saturation levels needed to treat sepsis and septic shock.
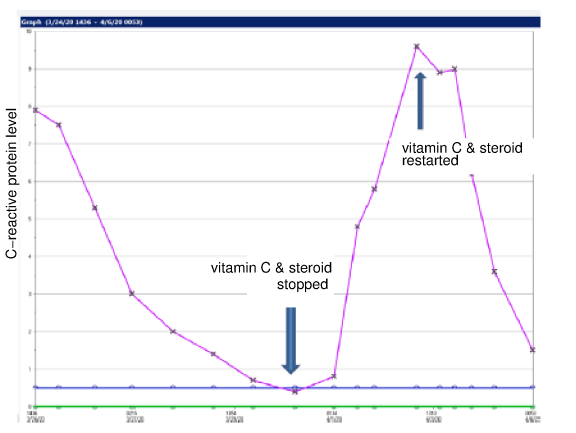
Is 70 years of successful treatments to thousands of patients insufficient evidence? If more studies are needed, who will put the 350-700 mg/kg/day IVC dose to the test without the dangerous and artificial 96 hour limitation?
I would like to acknowledge Benjamin Rakotoambinina, MD, PhD, professor of Physiology at the University of Antananarivo, Madagascar in collaboration with Laurent Hiffler, MD of the Cellular Nutrition Research Group for their critical review and feedback; and Drs. Robert G. Smith and Andrew Saul for their critical review and editorial support.
(Michael E. Passwater, son of author and columnist Dr. Richard Passwater, is certified by the American Society for Clinical Pathology as a medical technologist, a specialist in immunohematology, and is a diplomate in laboratory management. He has worked in clinical laboratories for 28 years, and has previously written “Do the Math: "MATH+" Saves Lives†published by the Orthomolecular Medicine News Service http://orthomolecular.org/resources/omns/v16n55.shtml ).
1. Sevransky JE, Rothman RE, Hager DN, et al. (2021) Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator-and Vasopressor-Free Days in Patients With Sepsis: The VICTAS Randomized Clinical Trial. JAMA 325:742-751. https://jamanetwork.com/journals/jama/fullarticle/2776688
2. Moraes RB, Friedman G, Wawrzeniak IC, et al. (2015) Vitamin D deficiency is independently associated with mortality among critically ill patients. Clinics. 70:326-332. https://pubmed.ncbi.nlm.nih.gov/26039948
3. Alker W, Haase H. (2018) Zinc and Sepsis Nutrients 10:976. https://pubmed.ncbi.nlm.nih.gov/30060473
4. Noormandi A, Khalili H, Mohammadi M, et al. (2020) Effect of magnesium supplementation on lactate clearance in critically ill patients with severe sepsis: a randomized clinical trial. Eur J Clin Pharmacol 76:175-184. https://pubmed.ncbi.nlm.nih.gov/31814044
5. Velissaris D, Karamouzos V, Pierrakos C, et al. (2015) Hypomagnesemia in critically ill sepsis patients. J Clin Med Res 2015;7:911-918. https://pubmed.ncbi.nlm.nih.gov/26566403
6. Guerin C, Cousin C, Mignot F, et al. (1996) Serum and erythrocyte magnesium in critically ill patients. Intensive Care Med 22:724-727. https://pubmed.ncbi.nlm.nih.gov/8880238
7. Angstwurm MW, Engelmann L, Zimmermann T, et al. (2007) "Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock." Crit Care Med. 35:118-26. https://pubmed.ncbi.nlm.nih.gov/17095947
8. Belsky JB, Wira CR, Jacob V, et al. (2018) A review of micronutrients in sepsis: the role of thiamine, l-carnitine, vitamin C, selenium and vitamin D. Nutr Res Rev. 31:281-290. https://pubmed.ncbi.nlm.nih.gov/29984680
9. Klenner FR. (1971) Observations On the Dose and Administration of Ascorbic Acid When Employed Beyond the Range of A Vitamin In Human Pathology. J Applied Nutrit. 23:61-87. https://seanet.com/~alexs/ascorbate/197x/klenner-fr-j_appl_nutr-1971-v23-n3&4-p61.htm
10. Chambers R, Pollock H. (1927) Micrurgical studies in cell physiology: IV. Colorimetric determination of the nuclear and cytoplasmic pH in th e starfish egg. J Gen. Physiol 10:739-755. https://pubmed.ncbi.nlm.nih.gov/19872358/
11. Clark EJ, Rossiter RJ. (1944) Carbohydrate metabolism after burning. Q J Exp Physiol Cog Med Sci 32:279-300. https://doi.org/10.1113/expphysiol.1944.sp000890
12. Fowler AA, Truwit JD, Hite RD, et al. (2019) Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 322:1261-1270. https://pubmed.ncbi.nlm.nih.gov/31573637
13. Fowler AA, Syed AA, Knowlson S, et al. (2014) "Phase I Safety trial of intravenous ascorbic acid in patients with severe sepsis." J Transl Med 12:32. https://pubmed.ncbi.nlm.nih.gov/24484547
14. DesBois M (2021) The Treatment of Infectious Disease Using Vitamin C and other Nutrients. Orthomolecular Medicine News Service. http://orthomolecular.org/resources/omns/v17n04.shtml
15. Klenner FR (1949) The Treatment of Poliomyelitis and other Virus Diseases with Vitamin C. South Med Surg. 111:209-214. https://pubmed.ncbi.nlm.nih.gov/18147027 https://vitamincfoundation.org/www.orthomed.com/polio.htm https://www.seanet.com/~alexs/ascorbate/194x/klenner-fr-southern_med_surg-1948-v110-n2-p36.htm
16. Jungeblut CW (1935) Inactivation of Poliomyelitis virus in vitro by crystalline vitamin C (ascorbic acid) J Exp Med. 62:517-521. https://pubmed.ncbi.nlm.nih.gov/19870431
17. Cathcart RF (1981) Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med Hypotheses 7:1359-1376. https://pubmed.ncbi.nlm.nih.gov/7321921
18. McCormick WJ (1951) Vitamin C in the Prophylaxis and Therapy of Infectious Diseases. Arch Pediatr. 68:1-9. https://pubmed.ncbi.nlm.nih.gov/14800557 https://www.seanet.com/~alexs/ascorbate/195x/mccormick-wj-arch_pediatrics-1951-v68-n1-p1.htm
19. Hugh D Riordan HD, Hunninghake RB, Riordan NH, et al. (2003) Intravenous ascorbic acid: protocol for its application and use. P R Health Sci J, 22:287-90. https://pubmed.ncbi.nlm.nih.gov/14619456
20. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. (2017) Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 151:1229-1238. https://pubmed.ncbi.nlm.nih.gov/27940189
21. Kory P, Meduri GU, Iglesias J, et al. (2021) Clinical and Scientific Rationale for the "MATH+" Hospital Treatment Protocol for COVID-19. J Intensive Care Med. 36:135-156. https://pubmed.ncbi.nlm.nih.gov/33317385
22. Front Line COVID-19 Critical Care Alliance (2021) EVMS COVID-19 Management Protocol: An overview of the MATH+ and I-MASK+ Protocols. http://www.flccc.net
23. Riordan H, Riordan, N, Casciari J (2021) The Riordan intravenous vitamin C (IVC) protocol for adjunctive cancer care: IVC as a chemotherapeutic and biologic response modifying agent. Riordan Clinic. https://riordanclinic.org/wp-content/uploads/2015/11/RiordanIVCprotocol_en.pdf
24. Heaney RP. (2014) Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 72:48-54. https://pubmed.ncbi.nlm.nih.gov/24330136
25. Montel-Hagen A, Kinet S, Manel N, et al. (2008) Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell, 132:1039-1048. https://pubmed.ncbi.nlm.nih.gov/18358815
26. Nualart F, Mack L, GarcÃa A, et al. (2014) Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. J Stem Cell Res Ther 4:209. https://pubmed.ncbi.nlm.nih.gov/25110615
27. May JM, Harrison FE. (2013) Role of Vitamin C in the Function of the Vascular Endothelium. Antioxidants & Redox Signaling 19:2068-2083. https://pubmed.ncbi.nlm.nih.gov/23581713
28. Nabzdyk CS, Bittner EA. (2018) Vitamin C in the critically ill - indications and controversies. World J Crit Care Med 7:52-61. https://www.wjgnet.com/2220-3141/full/v7/i5/52.htm
29. Lee RE. (1961) Ascorbic Acid and the Peripheral Vascular System. Ann NY Acad Sci. 92:295-301. https://doi.org/10.1111/j.1749-6632.1961.tb46129.x
30. Lee RE, Holze EA. (1951) Nutritional factors in hemodynamics: dissociation of pressor response and hemorrhage resistance in avitaminosis C. Proc. Soc. Expt. Biol Med. 76:325-329. https://pubmed.ncbi.nlm.nih.gov/14827915
31. Barabutis N, Khangoora V, Marik PE, Catravas JD. (2017) Hydrocortisone and Ascorbic Acid Synergistically Prevent and Repair Lipopolysaccharide-Induced Pulmonary Endothelial Barrier Dysfunction. Chest 152:954-962. https://pubmed.ncbi.nlm.nih.gov/28739448
32. Parker WH, Rhea EM, Qu ZC. (2016) Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am J Physiol Cell Physiol. 311:C652-C662. https://pubmed.ncbi.nlm.nih.gov/27605450
33. Gu W, Cheng A, Barnes H, et al. (2014) Vitamin C Deficiency Leading to Hemodynamically Significant Bleeding. JSM Clinical Case Reports 2:1046. https://www.jscimedcentral.com/CaseReports/casereports-2-1046.pdf
34. Zhao B, Fei J, Chen Y, et al. (2014) Vitamin C treatment attenuates hemorrhagic shock related multi-organ injuries through the induction of heme oxygenase-1. BMC Complementary and Alternative Medicine 14:442-454. https://pubmed.ncbi.nlm.nih.gov/25387896
35. Ladumer A, Schmitt CA, Schachner D, et al. (2012) Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med. 52:2082-2090. https://pubmed.ncbi.nlm.nih.gov/22542797
36. Heller R, Munscher-Paulig F, Grabner R, Till V. (1999) L-Ascorbic Acid Potentiates Nitric Oxide Synthesis in Endothelial Cells. J Biol Chem 274:8254-8260. https://pubmed.ncbi.nlm.nih.gov/10075731
37. Dingchao H, Zhduan Q, Xiaodong F. (1994) The Protective Effects of High-Dose Ascorbic Acid on Myocardium against Reperfusion Injury During and After Cardiopulmonary Bypass. Thorac Cardiovasc Surg 42:276-278. https://pubmed.ncbi.nlm.nih.gov/7863489
38. Ichim TE, Minev B, Braciak T, et al. (2011) Intravenous ascorbic acid to prevent and treat cancer-associated sepsis? J Transl Med 9:25. https://pubmed.ncbi.nlm.nih.gov/21375761
39. Cisternas P, Silva-Alvarez C, Martinez F, et al. (2014) The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J Neurochem 129: 663-671. https://pubmed.ncbi.nlm.nih.gov/24460956
40. Wang Y, Lin H, Lin BW, et al. (2019) Effects of different ascorbic acid doses on the mortality of critically ill patients: a meta-analysis. Ann Intensive Care 9:58. https://pubmed.ncbi.nlm.nih.gov/31111241
41. Boretti A, Banik BK. (2020) Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 12:100190. https://pubmed.ncbi.nlm.nih.gov/32322486
42. Iglesias J, Vassallo AV, Patel V et al. (2020) Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis. Chest 158:164-173. https://pubmed.ncbi.nlm.nih.gov/32194058
43. de Melo AF, Homem-de-Mello M. (2020) High-dose intravenous vitamin C may help in cytokine storm in severe SARS-CoV-2 infection. Crit Care 24:500. https://pubmed.ncbi.nlm.nih.gov/32792018
44. Zhang J, Rao X, Li Y et al. (2021) Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intenisve Care 11:5. https://pubmed.ncbi.nlm.nih.gov/33420963
45. Lankadeva YR, Peiris RM, Okazaki N, et al. (2021) Reversal of the pathophysiological responses to Gram-negative sepsis by megadose Vitamin C. Crit Care Med 49:e179-e190. https://pubmed.ncbi.nlm.nih.gov/33239507
46. Patterson G, Isales CM, Fulzele S. (2021) Low level of vitamin C and dysregulation of vitamin C transporter might be involved in the severity of COVID-19 infection. Aging and Disease 12:14-26. https://pubmed.ncbi.nlm.nih.gov/33532123
47. Tomassa-Irriguible TM, Lielsa-Berrocal L. (2020) COVID-19: Up to 87% critically ill patients had low vitamin C values. Research Square, preprint. https://www.researchsquare.com/article/rs-89413/v1
48. Arvinte C, Singh M, Marik PE. Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a North American Community Hospital Intensive Care Unit in May 2020. A pilot study. Medicine in Drug Discovery 8:100064. https://pubmed.ncbi.nlm.nih.gov/32964205
49. Wagas Khan HM, Parikh N, Megala SM, Predeteanu GS. (2020) Unusual Recovery of a Critical COVID-19 Patient After Administration of Intravenous Vitamin C. Am J Case Rep 21: e925521. https://pubmed.ncbi.nlm.nih.gov/32709838
50. Marik PE. (2018) Hydrocortisone, Ascorbic Acid and Thiamine (HAT therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients 10:1762. https://pubmed.ncbi.nlm.nih.gov/30441816
51. May JM, Qu ZC. (2011) Ascorbic acid prevents oxidant-induced increases in endothelial permeability. Biofactors 37:46-50. https://pubmed.ncbi.nlm.nih.gov/21328627
52. Utoguchi N, Ikeda K, Saeki K et al. (1995) Ascorbic acid stimulates barrier function of cultured endothelial cell monolayer. J Cell Physiol 163:393-399. https://pubmed.ncbi.nlm.nih.gov/7706381
53. Han M, Pendem S, Teh SL, et al. (2010) Ascorbate protects endothelial barrier function during septic insult: Role of protein phosphatase type 2A. Free Radic Biol Med 48:128-135. https://pubmed.ncbi.nlm.nih.gov/19840845
Orthomolecular medicine uses safe, effective nutritional therapy to fight illness. For more information: http://www.orthomolecular.org
To locate an orthomolecular physician near you: http://orthomolecular.org/resources/omns/v06n09.shtml
The peer-reviewed Orthomolecular Medicine News Service is a non-profit and non-commercial informational resource.
Albert G. B. Amoa, MB.Ch.B, Ph.D. (Ghana)
Seth Ayettey, M.B., Ch.B., Ph.D. (Ghana)
Ilyès Baghli, M.D. (Algeria)
Ian Brighthope, MBBS, FACNEM (Australia)
Gilbert Henri Crussol, D.M.D. (Spain)
Carolyn Dean, M.D., N.D. (USA)
Ian Dettman, Ph.D. (Australia)
Damien Downing, M.B.B.S., M.R.S.B. (United Kingdom)
Ron Erlich, B.D.S. (Australia)
Hugo Galindo, M.D. (Colombia)
Martin P. Gallagher, M.D., D.C. (USA)
Michael J. Gonzalez, N.M.D., D.Sc., Ph.D. (Puerto Rico)
William B. Grant, Ph.D. (USA)
Claus Hancke, MD, FACAM (Denmark)
Tonya S. Heyman, M.D. (USA)
Suzanne Humphries, M.D. (USA)
Ron Hunninghake, M.D. (USA)
Bo H. Jonsson, M.D., Ph.D. (Sweden)
Felix I. D. Konotey-Ahulu, MD, FRCP, DTMH (Ghana)
Jeffrey J. Kotulski, D.O. (USA)
Peter H. Lauda, M.D. (Austria)
Thomas Levy, M.D., J.D. (USA)
Alan Lien, Ph.D. (Taiwan)
Homer Lim, M.D. (Philippines)
Stuart Lindsey, Pharm.D. (USA)
Victor A. Marcial-Vega, M.D. (Puerto Rico)
Charles C. Mary, Jr., M.D. (USA)
Mignonne Mary, M.D. (USA)
Jun Matsuyama, M.D., Ph.D. (Japan)
Joseph Mercola, D.O. (USA)
Jorge R. Miranda-Massari, Pharm.D. (Puerto Rico)
Karin Munsterhjelm-Ahumada, M.D. (Finland)
Tahar Naili, M.D. (Algeria)
W. Todd Penberthy, Ph.D. (USA)
Zhiyong Peng, M.D. (China)
Isabella Akyinbah Quakyi, Ph.D. (Ghana)
Selvam Rengasamy, MBBS, FRCOG (Malaysia)
Jeffrey A. Ruterbusch, D.O. (USA)
Gert E. Schuitemaker, Ph.D. (Netherlands)
T.E. Gabriel Stewart, M.B.B.CH. (Ireland)
Thomas L. Taxman, M.D. (USA)
Jagan Nathan Vamanan, M.D. (India)
Garry Vickar, M.D. (USA)
Ken Walker, M.D. (Canada)
Raymond Yuen, MBBS, MMed (Singapore)
Anne Zauderer, D.C. (USA)
Andrew W. Saul, Ph.D. (USA), Editor-In-Chief
Associate Editor: Robert G. Smith, Ph.D. (USA)
Editor, Japanese Edition: Atsuo Yanagisawa, M.D., Ph.D. (Japan)
Editor, Chinese Edition: Richard Cheng, M.D., Ph.D. (USA)
Editor, French Edition: Vladimir Arianoff, M.D. (Belgium)
Editor, Norwegian Edition: Dag Viljen Poleszynski, Ph.D. (Norway)
Editor, Arabic Edition: Moustafa Kamel, R.Ph, P.G.C.M (Egypt)
Editor, Korean Edition: Hyoungjoo Shin, M.D. (South Korea)
Assistant Editor: Helen Saul Case, M.S. (USA)
Technology Editor: Michael S. Stewart, B.Sc.C.S. (USA)
Legal Consultant: Jason M. Saul, JD (USA)
Comments and media contact: editor@orthomolecular.org OMNS welcomes but is unable to respond to individual reader emails. Reader comments become the property of OMNS and may or may not be used for publication.
To Subscribe at no charge: http://www.orthomolecular.org/subscribe.html
To Unsubscribe from this list: http://www.orthomolecular.org/unsubscribe.html

This website is managed by Riordan Clinic
Information on Orthomolecular.org is provided for educational purposes only. It is not intended as medical advice.
A Non-profit 501(c)(3) Medical, Research and Educational Organization
3100 North Hillside Avenue, Wichita, KS 67219 USA
Phone: 316-682-3100; Fax: 316-682-5054
© (Riordan Clinic) 2004 - 2017
Consult your orthomolecular health care professional for individual guidance on specific health problems.